
Get ahead of your
Crohn's disease
progression
By understanding your potential risk for serious complications*, you and your healthcare provider (HCP) can make more informed decisions about your care. That's the power of being proactive.
*CDPATH defines serious complications as any fistulas or strictures in your bowels or any surgery in your bowels other than the area in or around the anus.
Patient Portrayal
Better understanding of your potential risk
CDPATH is an innovative tool for Crohn’s disease. It combines an analysis of your blood's biomarkers and genetic information with your HCP's evaluation of your disease to estimate the likelihood you are at a low, medium, or high risk for developing serious Crohn's disease-related complications* within 3 years.
To help you better understand the results, the CDPATH report comes with a clear and straightforward visual graph to show your estimated risk over a 3-year period.
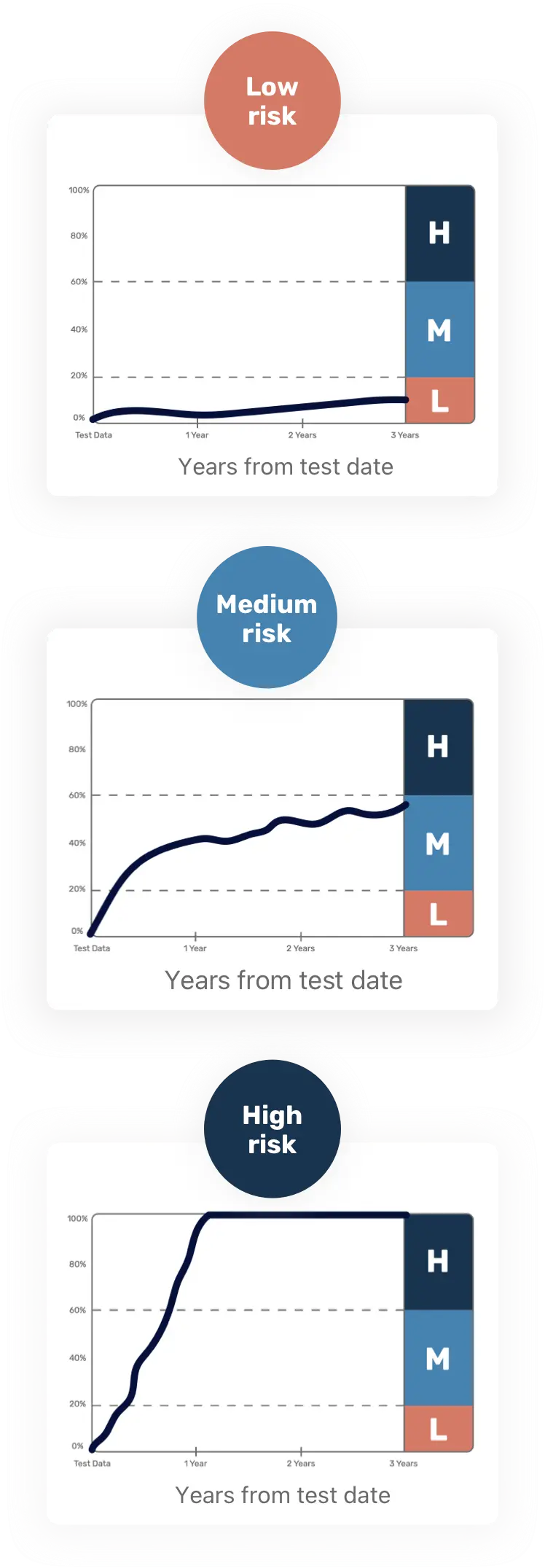
CDPATH results are prognostic but they are not a conclusion or guarantee. You and your HCP should not rely solely on the risk predictions from CDPATH to make a clinical diagnosis or treatment decision.
Actual charts will vary based on each individual patient's results.
Better collaboration with your healthcare provider
Crohn's disease is chronic, unpredictable, and can often feel overwhelming. Flares can happen without warning and symptoms can vary by individual.
Your CDPATH risk profile can help you and your HCP better understand your Crohn's disease and have more informed conversations about how to manage it more proactively.
Better planning through personalized, predictive insights
The earlier you and your HCP understand your potential risk for serious complications*, the better you can create a plan to proactively manage your disease.
